PFAS are a large group of manmade organofluorine compounds (>5,000) first developed in the early 1940s whose chemical structure gives them unique properties, including the ability to reduce friction and make products more resistant to stains, grease, water, and temperature. These chemical properties make them useful components in a wide array of industrial and commercial products such as textiles and leather products, metal plating, the photographic industry, photolithography, semi-conductors, paper and packaging, coating additives, non-stick cookware, food packaging, waterproof clothing, fabric stain protectors, lubricants, cleaning products, pesticides, paints, and aqueous film-forming foams (AFFF) used in firefighting.
Although there are thousands of PFAS compounds, certain PFAS have received particular regulatory attention. These key PFAS compounds are differentiated by the number of carbon atoms in the molecule and the presence of either a sulfonate or carboxylate functional group.
- Early regulatory attention: Perfluorooctane sulfonate (PFOS) and Perfluorooctane carboxylante (PFOA)
- Current regulatory attention in several States: PFHxS, PFBS, PFNA, PFHpA, PFDS, PFNS, PFHpS, PFPeS, PFTeDA, PFTrDA, PFDoA, PFUnA, PFDA, PFHxA, PFPeA, PFBA
- Increasing and Future attention: Fluorotelomers and all other PFAS
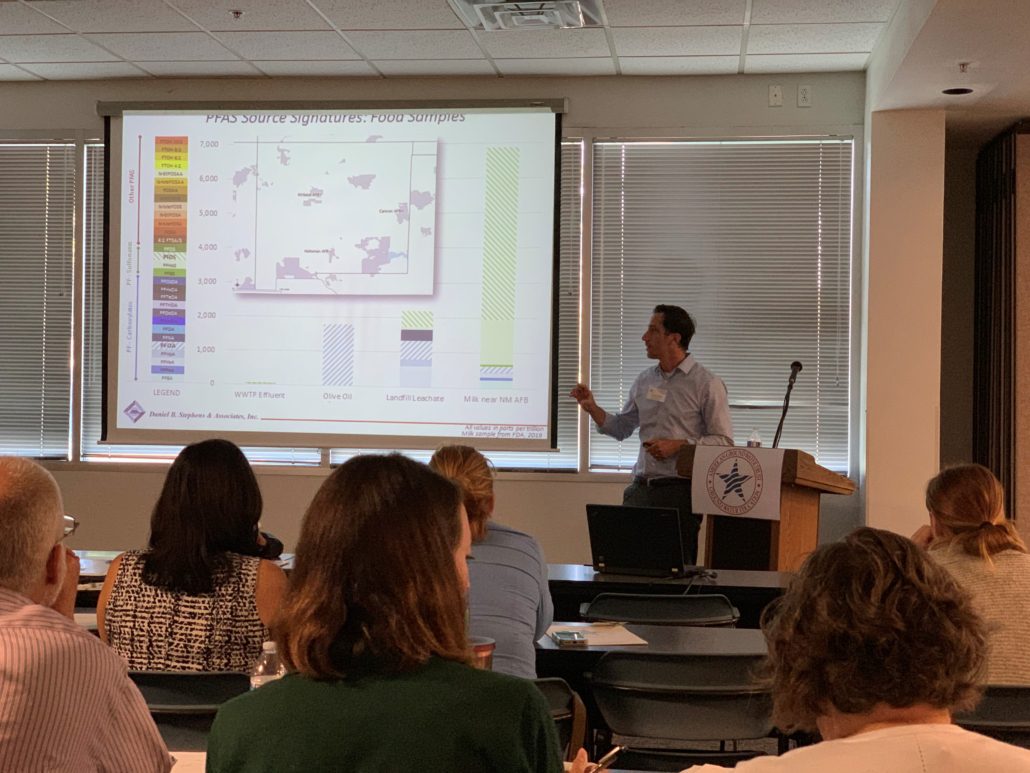
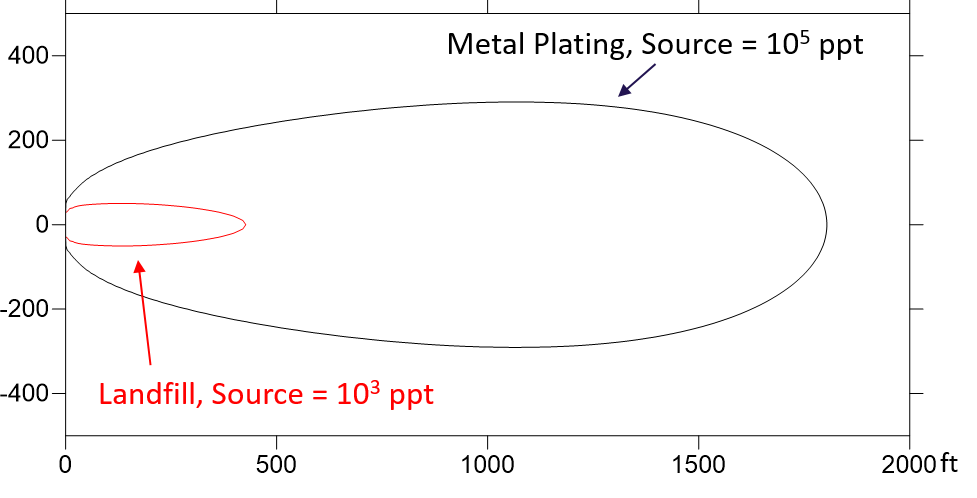
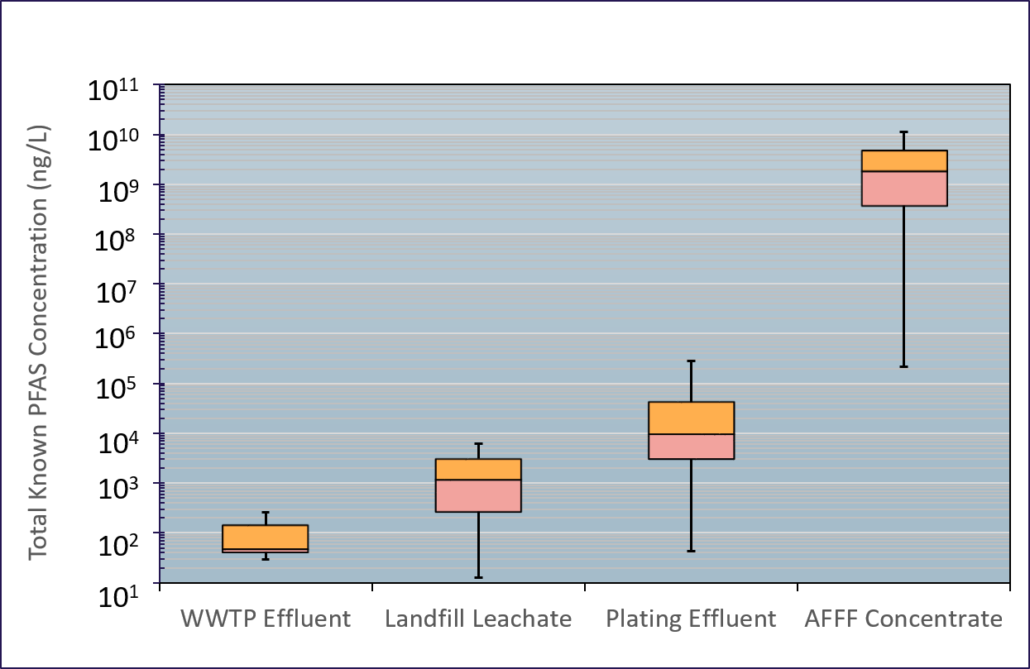
Sources of PFAS to the environment include waste-water treatment plant effluent, biosolids, septic systems, landfill leachate, metal plating facility effluent, ski wax, PFAS production facilities, and AFFF. DBS&A has characterized the chemical signature and total concentration of PFAS expected from these various sources, which vary significantly.
PFAS are extremely persistent in environmental media because of the highly stable carbon-fluorine bond. PFAS are resistant to environmental degradation processes and exhibit moderate sorption to soils and sediments dependent on the individual compound properties. PFAS typically have low volatility, but can adsorb to particles and be transported long distances in air. Because different PFAS transport at different rates, the distribution of various PFAS compounds will change from the source release to a receptor some distance away such as a groundwater production well.
In May 2016, the USEPA issued lifetime drinking water health advisories (HAs) for perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) of 70 nanograms per liter (ng/L), which applies to PFOS and PFOA individually or in combination. In February 2020, USEPA announced a proposal to regulate PFOS and PFOA under the Safe Drinking Water Act (which would result in a federal maximum contaminant level [MCL]) and requesting information and data on other PFAS substances.
Several states have also taken action to regulate PFAS. For example:
- The New Mexico Environment Department (NMED) has established preliminary soil screening levels (SSLs) for PFOA, PFOS, and perfluorohexanesulfonic acid (PFHxS) in residential, industrial, and construction worker exposure scenarios at 1.56, 2.60, and 7.08 milligrams per kilogram, respectively. NMED has also established a preliminary screening level for these three PFAS compounds in tap water at 70 ng/L. This preliminary screening level applies to each compound individually or in combination.
- California has established notification levels of 6.5 and 5.1 ng/L for PFOS and PFOA in drinking water, respectively, and response levels of 40 and 10 ppt for PFOS and PFOA.
- Michigan has established MCLs for seven PFAS, including an MCL of 16 and 8 ng/L for PFOS and PFOA, respectively.